Embibe Experts Solutions for Chapter: Kinetic Theory of Gases and Thermodynamics, Exercise 2: Exercise 2
Embibe Experts Physics Solutions for Exercise - Embibe Experts Solutions for Chapter: Kinetic Theory of Gases and Thermodynamics, Exercise 2: Exercise 2
Attempt the practice questions on Chapter 11: Kinetic Theory of Gases and Thermodynamics, Exercise 2: Exercise 2 with hints and solutions to strengthen your understanding. Practice Book for KVPY Aptitude Test - Stream SA Physics solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Kinetic Theory of Gases and Thermodynamics, Exercise 2: Exercise 2 with Hints & Solutions
The heat capacity of one mole of an ideal gas is found to be where is a constant. The equation obeyed by this gas during a reversible adiabatic expansion is-
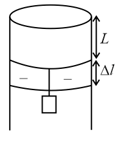
A long cylindrical pipe of radius is closed at its upper end and has an airtight piston of negligible mass as shown. When a mass is attached to the other end of the piston, it moves down. If the air in the enclosure is cooled from temperature to , the piston moves back to its original position. Then is close to (Assuming air to be an ideal gas, , atmospheric pressure is ),
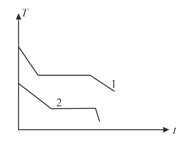
Two different liquids of same mass are kept in two identical vessel, which are placed in a freezer that extracts heat from them at the same rate causing each liquid to transform into a solid. The schematic figure below shows the temperature vs time plot for the two materials. We denote the specific heat in the liquid states to be and for materials and respectively, and latent heats of fusion and respectively. Choose the correct option.
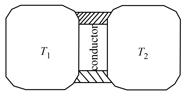
A thin piece of thermal conductor of constant thermal conductivity insulated on the lateral sides connects two reservoirs which are maintained at temperatures and as shown. Assuming that the system is in steady state, which of the following plots best represents the dependence of the rate of change of entropy on the ratio of temperatures
Two bottles and have radii and and heights and respectively with and . These are filled with hot water at . Consider that heat loss for the bottles takes place only from side surfaces. If the time the water takes to cool down to is and for bottles and , respectively, then and are best related as,
The number of gas molecules striking per second per square meter of the top surface of a table placed in a room at and atmospheric pressure is of the order of ( and the average mass of an air molecule is )
One mole of an ideal monatomic gas undergoes the following four reversible processes:
Step 1: It is first compressed adiabatically from volume to .
Step 2 : then expanded isothermally to volume
Step 3 : then expanded adiabatically to volume
Step 4 : then compressed isothermally to volume .
If the efficiency of the above cycle is then is
A gas obeying the equation of state undergoes a hypothetical reversible process described by the equation, , where and are dimensioned constants. Then, for this process, the thermal compressibility at high temperature
